Doe V. Rumsfeld: The Redux
Mandates? We've been here before...
Recently former Lieutenant Colonel, U.S. Army, Brad Miller published an article in reference to the military’s Covid-19 mandate.
His opening sentence:
“The Department of Defense’s (DoD) August 24, 2021 Covid-19 vaccine mandate was unlawful. That sentence…needs no additional context or qualifier to make it true. It is true as stated”
Spoiler: Brad does in fact give contextual background information.
However, I want to zoom in on this idea that the Covid-19 mandate was unlawful, analyzing Doe v. Rumsfeld as the case series that sets the legal precedent.
The “vaccine” bait-and-switch
To understand why Doe v. Rumsfeld is so relevant in the covid environment, it is worth revisiting the vaccine bait-and-switch saga.
There has been a wealth of information online describing how the Biden Administration mandated an unlicensed Emergency Use Authorized (EUA) product as if it was a licensed product.
However…
An Emergency Use Authorization is a separate legal classification from a product that receives Full Licensure. As products that carry separate legal classifications, they also carry separate requirements as well as separate liability protections. Regardless of any similarities between any two products, the two classifications are legally distinct. That legally distinct language comes directly from the FDA.
The best place to learn about EUA’s online is at the Association of State and Territorial Health Officials’s (ASTHO) Emergency Use Authorization Toolkit. There you will find several short facts sheets that address the fundamental issues and legal authorities for EUAs.
If you are new to the bait-and-switch conversation see the list of resources below. If not skip ahead!
List of Resources:
Congressional Letter to SECDEF led by Congressman Mark Green citing Doe v. Rumsfeld dated Aug 6, 2021
CHD Announces lawsuit against FDA for bait-and-switch by The Defender dated Sept 7, 2021
Assistant Secretary of Defense Health Affairs Interchangeability memo by Terry Adirim dated Sept 14, 2021
Attempt to correct Adirim’s memo by Dr. David Smith dated Oct 20, 2021
Declaration of Peter Marks, M.D, Ph.D., Director of FDA’s CBER division; Exhibit 13 dated Oct 21, 2021. Coker v. Austin. See: Paragraphs 6-11.
Shell Game? There remains no FDA approved COVID vaccine in the United States by Jordan Schachtel dated Dec 29, 2021.
The Comirnaty Project - Video published by Feds for Medical Freedom dated April 4, 2022
Nine Military Officers’ Whistleblower Report dated 15 August 2022. Condensed version. Full version: here.
Frankly, the list can go on but the KEY TAKEAWAY is that neither Pfizer nor Moderna have ever produced a fully licensed product. See: Dailymed Sept 13, 2021 NIH Announcement.
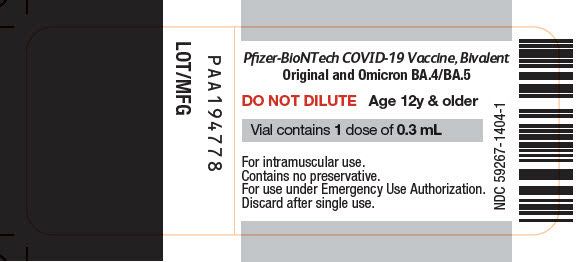
This is a crucial element to understand before analyzing Doe v. Rumsfeld. It is also worth noting that the currently available bivalent vaccines are classified under the Emergency Use Authorization. See labels below:
If you skipped, start here:
The Question then is, Can the Government Mandate an EUA Product?
If you were to ask the current administration then they would tell you yes.
Hosted on DoJ’s website is what is called a Slip Opinion authored by Dawn Johnsen of the Attorney General’s Office dated July 6th 2021 (~1.5months before the military mandate). In this slip opinion, Johnsen argues that EUA products can be mandated.
Her opinion is full of legal contradictions and can be considered a hostile interpretation of the law. But how much weight should be given to this piece of writing? The Slip Opinion is nothing more than exactly that: an opinion. Its only relevance is to provide an insight into the philosophies that were prevalent at the White House. The recipient of Dawn Johnsen’s opinion was the Deputy Counsel to the President—which provides legal advice to the President.
The advice in the opinion went unfollowed. The Biden Administration did not mandate an unlicensed EUA drug. They instead mandated the licensed version, never produced it, and continued to use the unlicensed version to force compliance with the mandates. See Bait-and-Switch discussion above.
In contrast to the Johnsen opinion, a better and more correct interpretation of the law can be found here. It is a letter from the Siri | Glimstad law firm dated Aug 4th, 2021 responding to Dawn Johnsen. Here’s a glimpse:
“In your Slip Opinion, you assert that expulsion from a job, school, and civil society are only “secondary consequences” which does not remove the “option to accept or refuse.” Not only does this argument defy common sense, but Section 564’s history, statutory framework, and implementation all reflect that “the option to accept or refuse” was intended to continue the longstanding principle that it is not permissible to coerce anyone to receive an unlicensed medical product.”
And another one:
“In sum, your reading of Section 564 as a requirement that an individual be informed that they have a “choice” while at the same time allowing the product to be mandated is illogical and contrary to the plain meaning, intent, and history of Section 564.”
Something that Dawn Johnsen does in her slip opinion is, she very narrowly only addresses the most recent covid lawsuits when asking if an EUA product can be mandated. In that narrow focus, she claimed “the only judicial decision to have addressed this issue [EUA mandates] so far summarily rejected the challenge. See Bridges v. Houston Methodist Hosp.” (As of publishing this case is still active. No final decision has been made).
However, the issue has been addressed almost 20 years ago during the DoD’s Anthrax Vaccine Immunization Program (AVIP).
Anthrax and Covid Parallels
If you are completely new to Anthrax, there is so much to discover. The anthrax saga is one of the more fascinating stories I have had the pleasure of learning about. Anthrax has a deep and captivating history and it’s often difficult to know where to begin. I will recommend two reads for now; one from the military perspective (Tom Rempfer) and one from the medical perspective (Dr. Meryl Nass).
Anthrax Vaccine Immunization Program Process Analysis by Rempfer & Dingle (undated). Be sure to read the footnotes.
The Anthrax Vaccine Progam: An Analysis of the CDC’s Recommendations for Vaccine Use by Meryl Nass, MD Dated May 2002.
To highlight some of the parallels:
President tasks SECDEF to mass vaccinate the troops
Then: Clinton to Cohen (1997)
Now: Biden to Austin (2021)
Safety and efficacy not established for the desired use
Substandard Vaccine Manufacturing Practices
Then: Good Manufacturing Practices requirement not met
Now: Good Manufacturing Practices waived
Physician judgment suppressed
Then: Military doctors
Now: All doctors
Pressure from senior leaders
Then: Cohen “I would be derelict in my duty not to vaccinate the troops." General Officer helmet analogy – "This is no different than an order to wear a helmet."
Now: Austin “All FDA-authorized Covid-19 vaccines are safe and highly effective. They will protect you and your family. They will protect your unit, your ship, and your co-workers. And they will ensure we remain the most lethal and ready force in the world. Get the shot. Stay healthy. Stay ready.”
The list goes on.
Enter Doe v. Rumsfeld
To set the stage, I’ll quote from Meryl Nass’ article above:
“In March 1998, Secretary Cohen was publicly vaccinated, and mandatory mass vaccinations began. The science to support the program did not exist. There were no published studies documenting the safety or efficacy of this vaccine for any route of exposure in humans, although human studies are required under the Food, Drug and Cosmetic Act. Within weeks, military service members began reporting illnesses following vaccination, while others refused the vaccine. The military leadership responded with court-martials, fines, and less-than-honorable discharges.”
John Doe #1 et al v. Rumsfeld et al was filed on March 18th, 2003 in the District of Colombia District Court (Case #1:03-cv-00707).
Mark Zaid, one of the attorneys representing the plaintiffs on that case has gathered the relevant opinions on his website, making it really easy to find and digest the information.
Below is a summary of the three important decisions from that case.
On December 22, 2003 (297 F. Supp. 2d 119 (D.D.C. 2003), Judge Emmet G. Sullivan granted the Motion for Preliminary Injunction citing that the military was to be enjoined from inoculating service members without their consent, absent a presidential waiver.
Despite the anthrax vaccine (AVA) achieving licensure in the 1970s, the military was using it for an unapproved purpose—to protect against aerosolized anthrax. The use of AVA against inhalation anthrax had never been tested. Therefore, for such a purpose, AVA was considered an Investigational New Drug (IND). By mandating the use of an IND, the Judge found that the DoD was in violation of 10 U.S.C 1107, Executive Order 13139, and DoD Directive 6200.2.
In this opinion, Judge Sullivan stated:
“The women and men of our armed forces put their lives on the line every day to preserve and safeguard the freedoms that all Americans cherish and enjoy. Absent an informed consent or presidential waiver, the United States cannot demand that members of the armed forces also serve as guinea pigs for experimental drugs.”
8 Days after the above decision, the FDA published a Final Rule and Order classifying AVA as a Category I Drug. In that Final Rule and Order, the FDA claimed that AVA was safe and effective “independent of the route of exposure.” Meaning that suddenly and despite the lack of evidence, the FDA claimed that AVA will in fact protect humans against inhalation anthrax.
On October 27, 2004 (341 F. Supp. 2d 1 (D.D.C. 2004) Judge Sullivan not only vacated and remanded the FDA’s Final Rule and Order but also granted a Permanent Injunction. This decision is a must-read!
Decision Highlights:
The “Brachman Study” - Calculated the effectiveness of the anthrax vaccine at 92.5% protection only against cutaneous anthrax and that inhalation anthrax occurred too infrequently to assess the protective effect of the vaccine against inhalation anthrax.
To make matters worse, the study was completed in 1962 based on the predecessor to the AVA vaccine licensed in the seventies. In other words, the vaccine being used by the military and the vaccine in the study were not the same product.
Dr. Nass more accurately describes the study stating:
“The quoted 92.5% vaccine efficacy figure was derived from a study of an unlicensed, precursor anthrax vaccine. It is also incorrect, having been calculated by improperly excluding one or more of the vaccinated participants who later developed anthrax”
The Brachman Study was used to justify the FDA’s Final Rule and Order in 2003.
The “Proposed Rule and Order of 1985” - Before the Final Rule of 2003, the FDA issued a proposal in the mid-eighties to classify AVA as a Category I drug. The proposed rule adopted the findings from the Brachman Study verbatim.
The proposal also required public comment. 4 comments were made, but none of them addressed the reclassification of AVA.
Citizen Petition of 2001 - The petition was to request that the FDA declare AVA ineffective for use against inhalation anthrax and to issue a Final Order to classify AVA as a Category II product.
In response, the FDA explained that they were working to complete the final rulemaking as soon as possible and given the urgency, it could not evaluate the adequacy of the petition.
8 Days after the preliminary injunction and 18 years after the Proposal rule… FDA issued the Final Rule
In short, the Court was not convinced:
“Accordingly, it is by the Court hereby ORDERED that Plaintiff's Motion for Summary Judgment is GRANTED. The FDA's Final Rule and Order is vacated and shall be remanded to the agency for reconsideration in accordance with this Memorandum Opinion and Order. Unless and until FDA properly classifies AVA as a safe and effective drug for its intended use, an injunction shall remain in effect prohibiting defendants' use of AVA on the basis that the vaccine is either a drug unapproved for its intended use or an investigational new drug within the meaning of 10 U.S.C. § 1107. Accordingly, the involuntary anthrax vaccination program, as applied to all persons, is rendered illegal absent informed consent or a Presidential waiver…”
With this opinion, AVIP was declared illegal, the preliminary injunction became permanent, and AVA was found to be neither safe nor effective against inhalation anthrax due to a lack of properly conducted studies.
Now, look at my shot record for dramatic effect:
If the involuntary program was declared illegal… how did I get injected 8 times without informed consent? Did AVA finally get approved for use for the military’s intended purpose or was there something else going on?
The answers to those questions are found in the third decision dated April 6, 2005. It’s a relatively short decision (2 pages). It’s worth posting the opinion verbatim:
ORDER
On October 27, 2004, this Court issued an order permanently enjoining the military’s anthrax vaccine program. Specifically, the Court held, “Unless and until FDA classifies AVA as a safe and effective drug for its intended use, an injunction shall remain in effect prohibiting defendants’ use of AVA on the basis that the vaccine is either a drug unapproved for its intended use or an investigational new drug within the meaning of 10 U.S.C. § 1107. Accordingly, the involuntary anthrax vaccine program, as applied to all persons, is rendered illegal absent informed consent or a Presidential waiver.”
Defendants have now filed an Emergency Motion to Modify the Injunction, seeking clarification that there exists a third option - an alternative to informed consent or a Presidential waiver - by which defendants can administer AVA to service members even in the absence of FDA approval of the drug: that is, pursuant to an Emergency Use Authorization (“EUA”) under the Project BioShield Act of 2004, 21 U.S.C.A. § 360bbb-3.
In enacting the EUA provision, Congress appears to have authorized the use of unapproved drugs or the unapproved use of approved drugs based on a declaration of emergency by the Secretary of Health and Human Services, which in turn is based on “a determination by the Secretary of Defense that there is a military emergency, or a significant potential for a military emergency, involving a heightened risk to United States military forces of attack with a specified biological, chemical, radiological or nuclear agent or agents.” 21 U.S.C.A. § 360bbb3(b)(1)(B).
Without ruling on the lawfulness or merits of any EUA, upon consideration of the defendants’ motion, the opposition and replies thereto, the amicus curiae brief, the arguments heard in open court on March 21, 2005, and the draft language jointly submitted by the parties in this case, it is hereby
ORDERED that the defendants’ Motion to Modify the Injunction is GRANTED; it is further
ORDERED that the Court’s injunction of October 27, 2004, is modified by the addition of the following language: “This injunction, however, shall not preclude defendants from administering AVA, on a voluntary basis, pursuant to the terms of a lawful emergency use authorization (“EUA”) pursuant to section 564 of the Federal Food, Drug, and Cosmetic Act, without prejudice to a future challenge to the validity of any such EUA. The Court expressly makes no finding as to the lawfulness of any specific EUA that has been or may be approved by the Department of Health and Human Services.”
Signed:Emmet G. Sullivan
United States District Judge
April 6, 2005
So the answer is no. AVA did not get approved for inhalation anthrax (at least not yet).
Rather than follow the Judge’s order to prove that AVA was safe and effective against inhalation anthrax, the DoD pressured the FDA to grant AVA the very first emergency use authorization on Feb 2, 2005:
“FDA is issuing this Authorization under the Federal Food, Drug, and Cosmetic Act (the act), as requested by DoD”
Within a few months of the EUA (thanks to the Project Bioshield Act of 2004), the DoD filed an emergency motion to continue inoculating service members against anthrax. The Judge granted the motion as seen above—so long as the vaccine requirement remained voluntary.
If the anthrax vaccine was voluntary because of the EUA, why did no one tell me?
The Aftermath
DoD pamphlet dated 5 April 2005.
Within a few months of the EUA issuance and one day before Judge Sullivan amended the Injunction, the DoD was already acknowledging that the EUA vaccine cannot be mandated. That service members have the right to refuse EUA and that the service member will not be punished.
Also there is a subtle warning and foreshadow dead center of the document: “ The issue of mandatory vaccination will be reconsidered after the FDA completes its administrative review, which DoD expects to occur later in 2005.
That is exactly what happened…
The FDA re-issued Final Rule and Order on December 29, 2004 (two months after the Permanent injunction) which went into effect on December 19, 2005. The stated purpose of this new final order was to “categorize AVA according to the evidence of its safety and effectiveness, thereby determining if it may remain licensed and on the market.” No new studies were used. Instead, the FDA simply “reviewed” the information from the original 1985 proposal—which was based on the Brachman study and an open label safety study conducted by the National Center for Disease Control. In this new 2004 proposal, the FDA simply did not agree with itself and the 1985 proposal
In its report, the Panel stated that the Brachman study results… that “inhalation anthrax occurred too infrequently to assess the protective effect of vaccine against this form of the disease” (50 FR 51002 at 51058, December 13, 1985). We do not agree with the Panel's statement that the protection was limited to cutaneous anthrax cases.
Looking back at the October 27, 2004 injunction, Judge Sullivan essentially told the FDA how to get around the injunction. “Unless and until FDA properly classifies AVA as a safe and effective drug for its intended use, an injunction shall remain in effect.” This is the statement that was quoted in the 2006 mootness decision.
On February 9, 2006 the U.S. Court of Appeals for the District of Columbia found that the issue had resolved itself due to the re-issued December 2005 Final Rule and Order and mooted the case.
Judge Sullivan summarizes the case here in an August 2007 filing.
While this case was on appeal, in December 2005, the FDA issued a new final order after a notice-and-comment period, explicitly finding AVA efficacious against inhalation anthrax. See Biological Products; Bacterial Vaccines and Toxoids; Implementation of Efficacy Review; Anthrax Vaccine Adsorbed, 70 Fed. Reg. 75,180 (Dec. 19, 2005). As a result, the D.C. Circuit held that this Court’s permanent injunction had dissolved by its own terms, dismissed the appeal as moot, and remanded the case to this Court for further proceedings. Doe v. Rumsfeld, 172 Fed. Appx. 327 (D.C. Cir. 2006) (per curiam). On remand, the only remaining issue is plaintiffs’ motion for attorneys’ fees and costs under the Equal Access to Justice Act (“EAJA”) 28 U.S.C. § 2412(d).
After the February 2006 mootness decision, AVIP went through a series of start stops due to new lawsuits against the program. There was a DoD announcement in October 2006 that vaccinations were to remain voluntary until further guidance. A new lawsuit was filed December 2006. By February 2007, the DoD had resumed mandatory vaccinations. This Washington Post article discussing the new lawsuit reads as if it was written today:
"We've never been anti-vaccine, we're just against them forcing people to take it," Zaid said. "This vaccine is just completely unscientifically justified based on its effectiveness or necessity.”
In another case (Rempfer et al v. Dept of Air Force Board for Correction of Military Records), Judge Robertson had affirmed Judge Sullivans prior decisions in March of 2008. However, Judge Robertson did not contest the FDA’s 2005 rule-making. Here’s the conclusion:
Taken as a whole, Judge Sullivan’s decisions in Doe v. Rumsfeld conclude that, prior to the FDA’s December 2005 rule-making, it was a violation of federal law for military personnel to be subjected to involuntary AVA inoculation because the vaccine was neither the subject of a presidential waiver nor licensed for use against inhalation anthrax.
Despite the FDA’s repeated regulatory malfeasance, it seems the 2005 rule-making did not receive the same level of scrutiny as the 2003 order. The difference between the 2003 and the 2005 rule-making are virtually non-existent. When comparing Judge Sullivan’s October 27, 2004 summary of the case proceedings and the 2005 rule-making, there were no significant studies or data available to justify the FDA’s declaration that AVA is safe and effective against inhalation anthrax. The only true difference between the 1985 rulemaking and subsequent rulemaking, was the political environment and the Panel’s interpretation of the available studies.
Recap of Significant Events:
December 13, 1985 - Proposed Rule and Order to classify AVA as a Category I drug.
March 1998 - DoD begins mandatory vaccinations. Shortly thereafter service members report illnesses.
September 30, 1999 - President Clinton signs E.O 13139 to vaccinate service members against the threat of biochemical warfare.
September 18, 2001 - Anthrax letter attacks (igniting the discussion to protect service members against BW attacks).
March 18, 2003 - Doe v Rumsfeld was filed.
December 22, 2003 - Judge Sullivan issued a Preliminary Injunction.
December 30, 2003 - FDA issued the Final Rule and Order of the 1985 Proposal with significant departures from the 1985 findings. FDA had declared AVA safe and effective against inhalation anthrax despite the 1985 findings that declare the contrary.
July 21, 2004 - President Bush signed Project Bioshield Act into law. This allowed Human Health Services Secretary the authority to grant emergency use authorizations.
October 27, 2004 - Judge Sullivan issued permanent injunction and declared AVIP illegal.
December 29, 2004 - FDA re-issued a Proposed Rule and Order without any significant changes.
Feb 2, 2005 - FDA granted AVA an EUA to protect against inhalation anthrax (thus signaling AVA is unapproved for use against aerosolized anthrax). Essentially a band-aid fix to allow DoD to continue inoculations.
April 5, 2005 - DoD publishes updated trifold pamphlet acknowledging AVA vaccination is voluntary.
April 6, 2005 - Judge Sullivan modifies the injunction based on the EUA provision that AVIP was to remain voluntary.
December 19, 2005 - FDA finalized the 2004 Proposal.
February 9, 2006 - Doe v Rumsfeld mooted based on the 2005 Final Rule and Order.
December 22, 2008 - FDA approved a new version of Emergent’s BioSolutions BioThrax. Press Release here. Inhalation anthrax is suspiciously not mentioned.
Conclusion
By the time I enter the Air Force in 2010, the anthrax legal battle was officially over. The final nail in the coffin was a 2009 D.C. Circuit Court of Appeals Decision which upheld a lower court’s decision to dismiss the case challenging the FDA’s 2005 Final Rule and Order. The 2008 dismissal stated:
The FDA did not act arbitrarily or capriciously. It considered the relevant data and articulated an explanation establishing a "rational connection between the facts found and the choice made." Bowen, 476 U.S. at 626. The Court will not substitute its own judgment when the FDA made no clear error of judgment.
Given the history of the case, it is difficult to agree with Judge Collyer’s decision to dismiss.
Instead, I see a story of agency capture, manipulation, and legal maneuvering, followed by a series of “quick-fixes” to accomplish an unprecedented vaccine program. Not unlike what was witnessed during the Covid-19 mandates. The difference however, is that DoD, FDA, HHS, CDC, etc. have had nearly two decades of lessons learned.
Rather than going through the licensure process (which takes an average of about 10 years), the previous administration issued EUA’s for the Covid-19 products. Given the legal precedent that EUA cannot be mandated, the current Administration relied on agency capture, manipulation, and legal maneuvering to make the claim that the licensed product (which was never made available) has the same formulation as the EUA product. Then the current administration took the position that because the two have the same formulation, the government had the right to mandate the product as if it was licensed.
Back to whether or not the Covid mandates were unlawful… it’s no question. If the government intended to mandate a licensed product, then a licensed product should have been provided. Instead, the government coerced the masses into believing the unlicensed product was its licensed counterpart.
Afterthought
There are a lot of parallels between AVIP and the Covid mandates. One in particular that stands out is DoD’s premonitions about licensure.
For Anthrax, it was the Feb 2005 Pamphlet, where DoD expected FDA to complete its administrative review in Dec 2005.
For Covid, it was SECDEF’s Aug 9th, 2021 “Message to The Force” which stated:
I want you to know that I will seek the President' s approval to make the vaccines mandatory no later than mid-September, or immediately upon the U.S. Food and Drug Administration (FDA) licensure, whichever comes first.
By way of expectation, public reporting suggests the Pfizer-BioNTech vaccine could achieve full FDA licensure early next month.
Given the uncanny parallels, it is no wonder that attorney Dale Saran was able to predict exactly how the mandates would go, as early as September 8, 2021. In a blog post titled: “The Medical Autocracy is Here – DoD Vax Mandate, Pt. 2” Dale states:
Now you’re going to get your immune system on a Pfizer End User Agreement, 4 month boosters, just like your Windows antivirus software updates. Except no vaccine can beat your immune system and no vaccine was going to ever beat 99.997% efficacy – that’s the Infection Fatality Rate for the 18-29 year old age group, which comprises most young servicemembers. The FDA will beat its chest about COVID fatalities and stomp around on the graves of 610,000 people who died “from” Covid, but say nothing about the statistics that really matter if we’re going to have adult public health discussions. 95% of those deaths were people with multiple “co-morbidities.” The median age of death for this virus is roughly 80; that’s a year and change longer than the current US average life-expectancy.
All of those statements would later be found to be true.
However, the point of that specific blogpost was to highlight that while many of us covid types think that anthrax was the predecessor to the current state of affairs, the truth is more disturbing. He describes a realization he had back in 2000 while researching for his recently published book:
“Our government has a rather long, deliberate, deceitful history of inflicting medical experiments on our troops with no accounting ever have taken place for any of it. These aren’t conspiracy theories, these are well-documented facts from Senate Reports, and even Supreme Court cases.”
If you are interested in learning more about AVIP, Dale Saran defended marines at their courts-martial for refusing the experimental anthrax vaccine. His book is a first hand retelling of the cases he litigated during the anthrax vaccine immunization program. You can find the book here (on amazon). Today Dale is ligating on behalf of over a thousand service members affected by the unlawful covid mandate. If you know an affected service member send them to https://militarybackpay.com/.






First off, thanks for the shout-out in your intro. Second, and more importantly, thanks for all the homework you've done on this.
Wow. The cadre at Chemical BOLC in Leonord wood told the students this back in 2007. They said- just wait until you get all the anthrax shots- that are not tested and approved. This was during the “history of service members as human Guinea pigs for chemical and biological agents- lesson